-
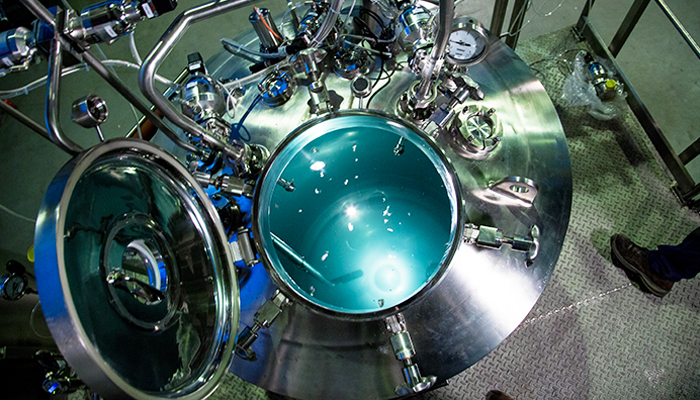
Liquids – Reactors and Preparation Vessels
Olsa is specialized in the process analysis, engineering, design, manufacturing and installation of preparation process equipment. With thousands of installations worldwide, Olsa has been contributing to develop the most advanced pharmaceutical and biotechnology processes partnering with major pharmaceutical companies around the world. Olsa engineers customize a wide range of products able to fulfill the specific needs of the most difficult production processes. The preparation vessels are designed and manufactured in compliance with the strictest cGMP guidelines both for sterile and non-sterile productions. Size range from 2 up to 50.000 liters matching EU cGMP & FDA requirements as well as ASME/PED certification.
- Aerosol
- API
- Eye drops
- SVP (Antibiotics, Oncological solutions, Blood derivates, Vaccines, Biological, Biotechnology products)
- LVP
- Dialysis solutions
- Syrups
- Suspensions
- Dispersions
-
Cake Filters / API Dryers
Filtration and drying are the most common and important technology used for the separation of solid from a liquid phase that will be subsequently be dried to reduce the humidity or the solvent content in the product. OLSA manufactures filters that recover the solid from the liquid phase. Then, the dryers remove the remaining solvent with many options depending the product characteristics from mix-dryers, static vacuum, hot air and dynamic dryers. Dryfilters are multipurpose units to perform in one single unit the product drying operation in addition to the soild-liquid separation.
- API intermediate and final active ingredients
- Animal and vegetable origin extracts
- Fine chemicals
-
Dryers
Hot air static dryers for parts and raw material. There are two types available: Hot air and vacuum. Hot air is the standard dryer designed to meet the widest requirements of the Pharmaceutical Industry. The vacuum dryers are suitable where high heat cannot be used either due to inflamable solvents or due to product sensitivity. DeLama dryers are designed for:
- powders and granulates
- empty glassware (e.g. vials, ampoules, bottles)
- inox equipment (e.g. trays, machine parts)
- thermosensitive material
- raw hyaluronic acid bulk production
- materials which require a low content of final residual humidity
-
Dry powder Blenders
Agierre designs and manufactures blenders for dry powders. Blenders are primary used in the Pharmaceutical and Chemical industries in all those processes of handling of powders in which there is the need to mix homogeneously the powders directly inside the bin without the loading, unloading and cleaning operations of the mixer. Blenders are available in standard blenders, blender columns, mobile systems and drum blenders.
-
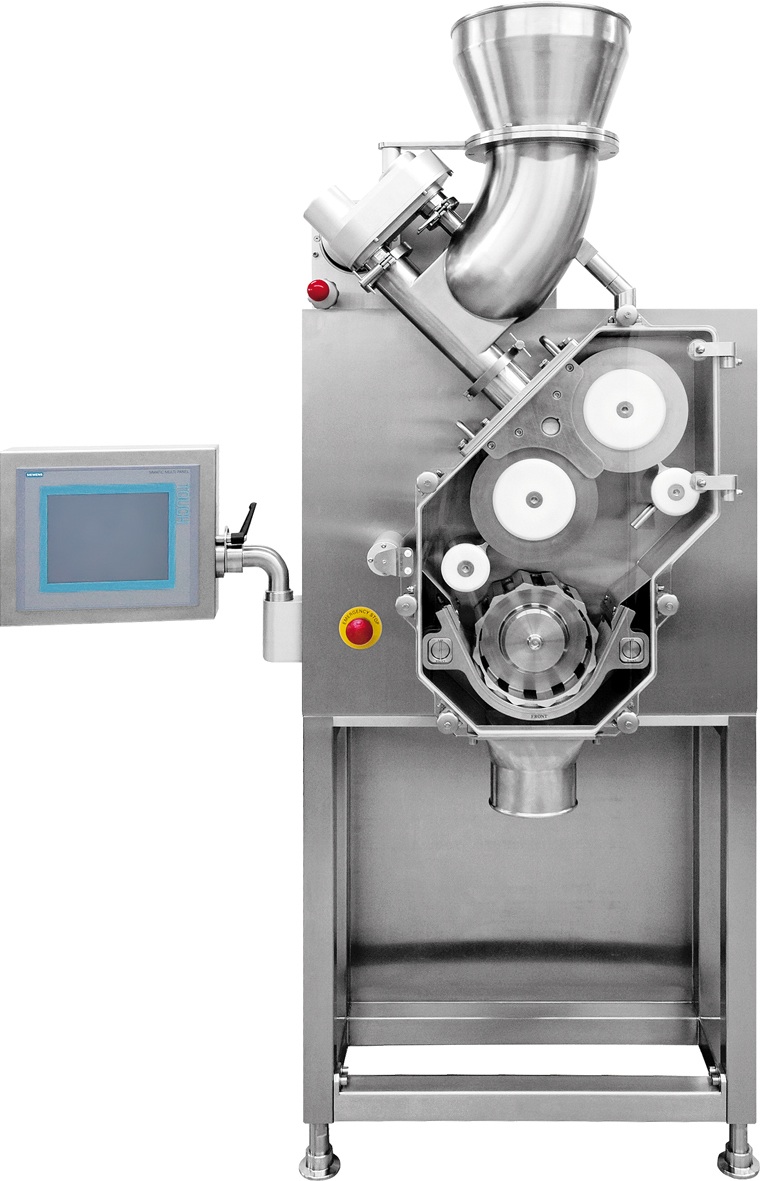
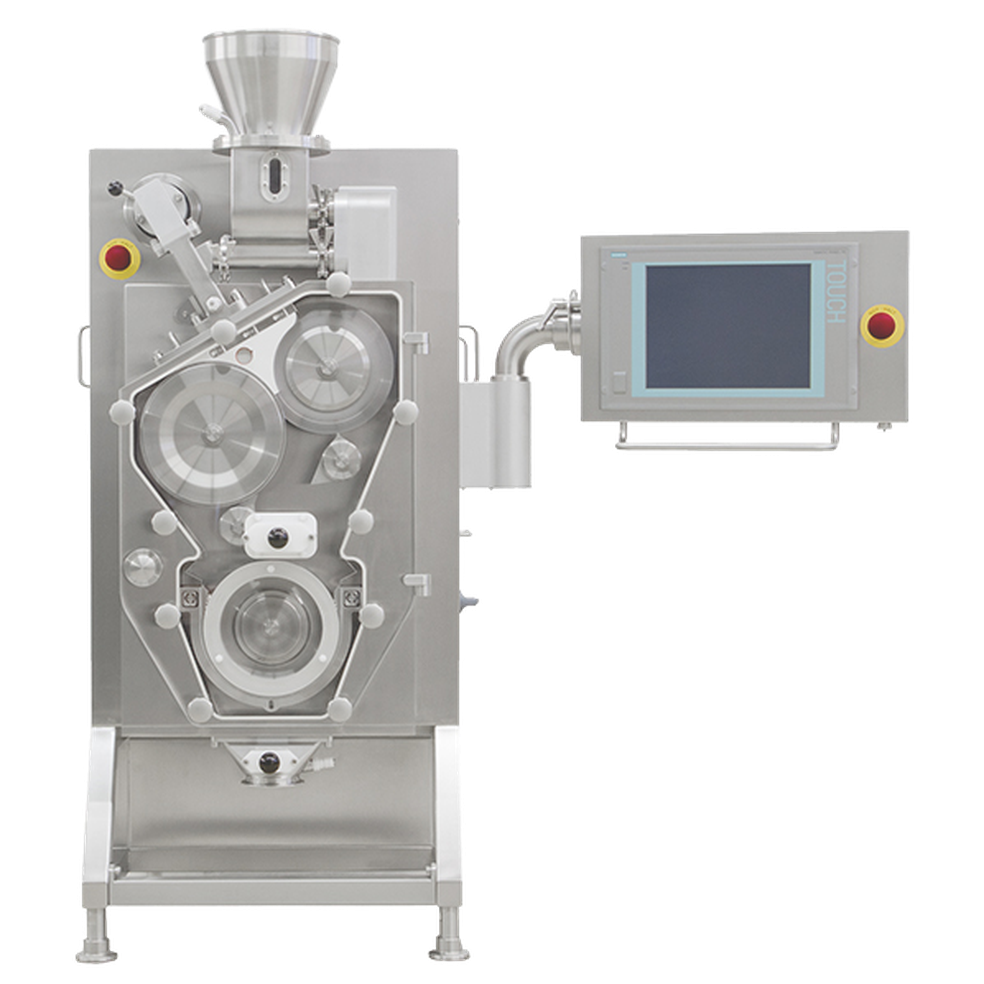
Roller Compactors and Granulators
Granulation is a process in which powder particles are made to adhere to each other, resulting in larger, multi-particle entities, so called granules. If such a process is performed without adding liquids, this is called dry granulation. In dry granulation, the powder blend is compacted by applying a force onto the powder, which in general causes a considerable size enlargement. The compacts obtained are called ribbons, flakes or briquettes. In order to obtain the desired granules, the compaction process is followed by a milling step. There are two methods for dry granulation: Slugging that uses a tablet press and Roller Compaction. Fine powder does not flow well into the die of a tablet press resulting in slugs with significant weight differences. With GERTEIS Roller Compaction we have a uniform weight and density on the resulting granules and at the same time minimizing the fines. The flow of the compacted granules is enhanced and controlled, suitable for high-speed tablet presses. In contrast to wet granulation, dry granulation is a truly continuous process. This results in various economic advantages. Currently the throughput of common dry granulation systems can reach approx. 400kg/h. the advantages of Dry Granulation are:
- Lower capital investment in facilities or equipment
- Low maintenance requirements resulting in drastically reduced lifecycle costs
- Minimal floor space
- Large throughput of material (highly efficient process)
- Truly continuous process
- Highly energy efficient; no heat for drying and no air conditioning required as for other granulation techniques
- Ultra High Containment Options are available to avoid contamination of manufacturing areas and personnel from high potent actives
-
Semi-solids
In the last 67 years Olsa has been the main partner for the most important pharmaceutical and cosmetic companies in the development of semisolid applications. Our know-how contributes to the development of the best processes for the production of creams, ointments, gels, toothpaste, nutraceutical and similar products. Throughout our internal testing and laboratory area, we are able to define and engineer the most suitable equipment for our clients, optimizing the shearing effects according to the different product needs. With this respect we are the only manufacturer of process mixing equipment that encompasses several different mixing systems such as: top coaxial agitators, bottom homogenizers, planetary top agitators, top homogenizers, internal recirculation and external recirculation.
- Ointments
- Suppositories
- Soft capsules
- Creams
- Lotions
- Gels
- Toothpastes
- Make-up
- Emulsions
- Cosmetic muds
- Jellies
- Baby Food
-
Bioprocess - Bioreactors
Biotechnology origin products are becoming more and more important in the Pharmaceutical Research, Development and Production areas. Our deep process know-how, extensive bioprocess expertise and excellent execution of highly sophisticated software grant a successful combination. This makes Olsa the right partner for the Biotechnology and Biological Operators, from R&D to industrial scale production. High Value Biotechnology products such as recombinant or biosimilar require a perfect level of specific competence and know how that is even more important considering that bioreaction is the core of the production process. Cell and bacterial cultivation units (Bioreactors) are designed and manufactured in compliance with the strictest cGMP guidelines and can be standard as well as customized. Product Range is from 2 up to 50.000 liters matching EU cGMP & FDA design as well as ASME/PED certification.
- Vaccines (Human and Veterinary)
- Recombinants
- Monoclonal Antibodies
- Antibiotics
- Hormones
-
CIP/SIP
Cleaning, Sterilizing and Drying in Place of Pharmaceutical, Biotechnology and Cosmetic equipment is the most appropriate and safe method for managing these critical and frequent operations. Our wide experience in engineering and manufacturing equipment and complex systems for liquid, semisolid and solid products has allowed us to design and manufacture the most appropriate and fully performing automated CIP/SIP/Drying units perfectly executing the different applications. CIP/SIP units are provided with one or two purified water buffer tanks, sanitary heat exchanger to heat up the washing solutions, detergent/chemical dosing groups in order to optimize the cleaning in place and conductivity meters, pH and TOC instruments to analyze the quality of washing solutions.
- Sophisticated control systems for automatic/semi-automatic operations/receipts in compliance with 21 CFR part 11, controlled by PLC and SCADA
- System design BPE/ASME, fully compliant with EU cGMP and FDA requirements
- Minimized water consumption during CIP cycles thanks to an optimized process design